Lumel : KD8
Screen recorder
KD8
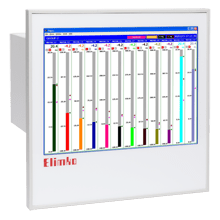
KD8 recorder is applied as a data acquisition station in measuring systems. It finds application to measure, visualize and supervise technical process parameters in various industrial branches, e.g. in pharmacy, food, chemical. KD8 recorder complies with the regulation 21 CFR Part 11, regulation for electronic records and signatures issued by Food and Drug Administration (FDA). MEASURING INPUTS and OUTPUTS 3 or 6 galvanically isolated analog measuring channels, 6 or 12 alarm outputs (2 for each chanel), 4 or 8 digital inputs. DATA EXPOSURE linear and bar trends, digital and analog indicators, statistics. Each channel has the possibility to assign settings as: colour, name, range and presentation view. To illustrate the process run, the customer can choose many forms of data presentation: DATA ARCHIVING For data archiving KD8 recorder has: exchangeable external memory (CompactFlash card), 6 MB internal memory with data support. WORK SAFETY To ensure the recorder work safety in the network, each customer can have individual login and password. PC SOFTWARE KD8 SETUP, KD ARCHIVE, KD CONNECT and KD CHECK programs are destined for KD8 recorder service. The KD8 SETUP program serves to configure KD8 recorders. The exchange of configuration data between the recorder and PC is carried out through the USB interface or the CompactFlash card. The KD ARCHIVE program is destined for the visualization, verification of the digital signature, printout and export to CSV format, data recorded in the binary format with digital signature, obtained from the recorder. The KD CONNECT program is destined for communication between PC and the KD8 recorder through the USB link. It enables the acquisition of archived data from the recorder, saving and erasing data on the CF card. The KD CHECK program is destined for verification of the digital signature in archive.
KD8 recorder is applied as a data acquisition station in measuring systems. It finds application to measure, visualize and supervise technical process parameters in various industrial branches, e.g. in pharmacy, food, chemical.
KD8 recorder complies with the regulation 21 CFR Part 11, regulation for electronic records and signatures issued by Food and Drug Administration (FDA).
MEASURING INPUTS and OUTPUTS
- 3 or 6 galvanically isolated analog measuring channels,
- 6 or 12 alarm outputs (2 for each chanel),
- 4 or 8 digital inputs.
DATA EXPOSURE
- linear and bar trends,
- digital and analog indicators,
- statistics.
Each channel has the possibility to assign settings as: colour, name, range and presentation view.
To illustrate the process run, the customer can choose many forms of data presentation:
DATA ARCHIVING
For data archiving KD8 recorder has:
- exchangeable external memory (CompactFlash card),
- 6 MB internal memory with data support.
WORK SAFETY
-
To ensure the recorder work safety in the network, each customer can have individual login and password.
PC SOFTWARE
KD8 SETUP, KD ARCHIVE, KD CONNECT and KD CHECK programs are destined for KD8 recorder service.
- The KD8 SETUP program serves to configure KD8 recorders. The exchange of configuration data between the recorder and PC is carried out through the USB interface or the CompactFlash card.
- The KD ARCHIVE program is destined for the visualization, verification of the digital signature, printout and export to CSV format, data recorded in the binary format with digital signature, obtained from the recorder.
- The KD CONNECT program is destined for communication between PC and the KD8 recorder through the USB link.
It enables the acquisition of archived data from the recorder, saving and erasing data on the CF card. - The KD CHECK program is destined for verification of the digital signature in archive.
All of our products ensure maximum reliability even in challenging working conditions and help your business operations run smoothly. The products we offer in industrial automation, energy management, cabling solutions, and many other areas adapt flexibly to the needs of different sectors.
Additionally, our products are manufactured using only high-quality materials and comply with international standards. Through the solutions we provide, we enable our customers to increase operational efficiency and optimize costs. Our company closely follows technological advancements and continuously offers innovative products to help our customers gain a competitive advantage.
On each of our product pages, you can find comprehensive information about technical details, areas of use, and product features. You can explore all the products you need to strengthen your industrial processes on our website and enjoy a seamless purchasing experience.
Similar Products
Can't find the product you're looking for?
LET US HELP YOU
Can't Find the Product You're Looking For? Let Us Know, and We'll Source It for You!
Are you searching for products not listed on our website or out of stock? Let us know your requirements, and our expert team will contact you as soon as possible to find the most suitable solution for you.